salt water freezing point chart|Salt and the freezing point of water – Science Projects : Baguio For saltwater, the boiling point is raised, and the melting point is lowered. By how much depends on how much salt there is. I’ll assume the salt is sodium chloride, NaCl (table . Diaz became a free agent after his fight last month at UFC 279. . An exhausted Ortiz stumbles to the finish line in his first 12-round fight. Lomachenko closes strong in a close fight that .
PH0 · Salt and the freezing point of water – Science Projects
PH1 · Salt Water Freezing Point Calculator
PH2 · Saline water
PH3 · Liquid
PH4 · Freezing Point of Water Compared to a Salt Solution
PH5 · Freezing Point of Saltwater
PH6 · Freezing Point of Salt Water
PH7 · Can Sea Water Freeze?
PH8 · Boiling and Freezing Points of Pure and Salty Water
PH9 · 13.8: Freezing
direct hiring, pogo hiring. pogo. pogo hiring. pogo, encoder, work from home. encoder. urgent hiring. direct hiring. work from home. non voice work from home. office staff. Return to Search Result Job Post Details. Data Entry Encoder - job post. Facilities Managers, Inc. 3.2 3.2 out of 5 stars.
salt water freezing point chart*******Ago 14, 2023 — Calculate the freezing point of salt water solutions using the cryoscopic constant and molality. Find FAQs about salt water freezing, desalting, and cooling .For saltwater, the boiling point is raised, and the melting point is lowered. By how much depends on how much salt there is. I’ll assume the salt is sodium chloride, NaCl (table .salt water freezing point chartFor saltwater that’s as saturated as it can possibly get (i.e. there’s no way to dissolve any more salt in it no matter how hard you tried), the freezing point is -21.1 degrees .
salt water freezing point chart Salt and the freezing point of water – Science ProjectsFor saltwater that’s as saturated as it can possibly get (i.e. there’s no way to dissolve any more salt in it no matter how hard you tried), the freezing point is -21.1 degrees .
Ene 30, 2023 — Up to the point where there is 23.3% of salt in the mixture, the more salt the lower the freezing point of the water. The second line is actually a solubility curve for salt in water - although it doesn't look quite .Peb 24, 2020 — The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. The freezing point of .Hul 12, 2023 — Because the freezing point of pure water is 0°C, the actual freezing points of the solutions are −22°C and −30°C, respectively. Note that \(\ce{CaCl_2}\) is substantially more effective at lowering the .The USGS salinity scale defines three levels of saline water. The salt concentration in slightly saline water is 1,000 to 3,000 ppm (0.1–0.3%); in moderately saline water is 3,000 to 10,000 ppm (0.3–1%); and in highly .How does salt melt ice or snow? Do other material do the same? Is salt creating heat to melt the snow? Is it changing the freezing point of water so water does not freeze at 0ºC (32º F)? In this project we will perform .
Seawater has unique properties: it is saline, its freezing point is slightly lower than fresh water, its density is slightly higher, its electrical conductivity is much higher, and it is .The freezing point of pure water is 0°C, but that melting point can be depressed by the adding of a solvent such as a salt. The use of ordinary salt (sodium chloride, NaCl) on icy roads in the winter helps to melt the ice from the roads by lowering the melting point of the ice. A solution typically has a measurably lower melting point than the .Hul 12, 2023 — This solute lowers the freezing point of the water, preventing the engine from cracking in very cold weather from the expansion of pure water on freezing. . The small increase in .At some point its freezing temperature will be the same as the freezer temperature, so the freezing will stop. You'll have some ice left, and some salty water. What is interesting is that this effect is used all over the place. Often, salt is put on roads to melt ice. . Salt water will only freeze if it gets cold enough. For water as salty as .
Freezing Point of Seawater vs. Pressure and Salinity. Related Topics Densities Densities of solids, liquids and gases. Definitions and convertion calculators. . Solubility of oxygen in equilibration with air in fresh water and seawater (salt water) - pressures ranging 1 - 4 bar abs. Salinity of Water Salinity - salt content - of fresh .
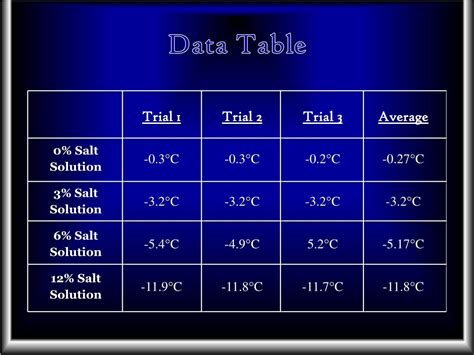
The amount of salt in water may be reported as the percent of salt in water or as grams of salt per liter of water. The dependent variable (also known as responding variable) is the freezing point of water. Freezing point is the temperature in which water will freeze. Constants are the amount of water in each cup.When you add salt to water, it lowers the freezing point of the water. Pure water freezes at 0°C (32°F), but saltwater freezes at a lower temperature. The salt particles disrupt the hydrogen bonding between the water molecules, making it harder for the water molecules to connect in an ordered way and form ice crystals, which slows down the .Peb 27, 2023 — The freezing point depression is directly proportional to the molality of the solute. . Water: 1.86.512: Acetic acid: 3.90: 3.07: Benzene: 5.12: 2.53: Phenol: . Since saltwater will freeze at colder temperatures, organisms can survive in these bodies of water. Exercise \(\PageIndex{1}\)Can Sea Water Freeze? Unit: Salinity Patterns & the Water Cycle l Time Required: Four 45 min. class period l Grade Level: Middle l Content Standard: NSES Physical Science, properties and changes of properties in matter Ocean Literacy Principle 1e: Most of Earth's water (97%) is in the ocean. Seawater has unique properties: it is saline, its freezing .The higher the concentration of salt, the lower the freezing point drops. Any foreign substance, such as sugar, alcohol, or any chemical salt, added to the water, forms a solute, which will lower the freezing point and melt ice. This is why salt is used to melt snow and ice on roadways and sidewalks. Salt is also inexpensive and readily available.
The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F). [ 1 ] The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier : the measured temperature was −2.6 °C (27.3 °F).Mar 14, 2024 — Some common examples of the freezing point depression phenomenon and its application in everyday life are: Salting of roads in winter: In winter, we use common salt (NaCl) or calcium chloride (CaCl 2) to clear the ice on the roads.This is because mixing salt with water lowers the freezing point of water below 0°C.As a result, water would .Yet according to the freezing point depression equation, the freezing point will rise during the experiment, why? Answer. As ice melts it turns to liquid water and the molaity goes down as the mass of liquid water .Hul 11, 2024 — The temperature of maximum density decreases faster than the freezing point as salt is added. At 24.70 psu salinity, the freezing point and the temperature of maximum density coincide at −1.332 °C (29.6 °F). At salinities typical of the open oceans, which are greater than 24.7 psu, the freezing point is always the temperature of .Up to the point where there is 23.3% of salt in the mixture, the more salt the lower the freezing point of the water. Note: If you are interested (although it isn't essential for understanding the current page), you can find out why salt lowers the freezing point of water by reading the page about Raoult's Law and non-volatile solutes .Brine Strength Pound/Gallon Brine Gram/Liter Brine Freezing Point* Salometer Degree Specific Gravity Baume Degree NaCl % Weight NaCl Water NaCl Water °F °C 0 1.000 0.0 0.00 .000 8.328 .0 998 + 32.0 0 2 1.004 0.6 0.53 .044 8.318 5.3 996 + 31.5 - 0.2 4 1.007 1.1 1.06 .089 8.297 10.6 995 + 31.1 - 0.5 . † Transition temperature from anhydrous .
Dis 31, 2020 — The freezing point of water depends on its pressure. (image: Cmglee, CC 3.0) Impurities also affect the freezing point of water. In nearly all cases, dissolving a substance (e.g., sugar, salt, alcohol) lowers the freezing point.Okt 3, 2022 — The freezing point of water depends on the salt concentration, with more salt, the freezing point is lower. The freezing point of salt water is -21.12 degrees Celsius for a concentration of 5 percent salt.
For pure compounds the following definitions can be given: Melting point - the temperature at which a solid turns into a liquid; Freezing point - the temperature at which a liquid turns into a solid ; The melting and freezing point changes .
Ganap nang inhinyero si Engr. Tomas Cabauatan Casauay Jr. matapos tumuntong sa Top 2 nationwide sa average na 92.60. Si Casauay ay tubong Tuguegarao City at nagtapos ng Bachelor of Science in Electrical Engineering sa Cagayan State University – Carig Campus.
salt water freezing point chart|Salt and the freezing point of water – Science Projects